That molecule is involved in 4 hydrogen bonds. Due to the strong hydrogen bonding the average NH.
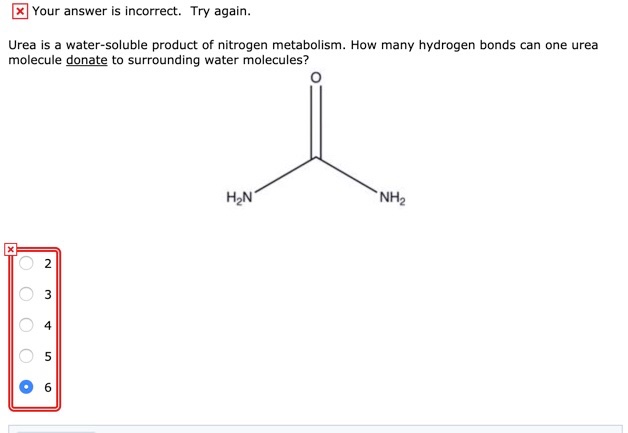
Solved X Your Answer Is Incorrect Try Again Urea Is A Chegg Com
When two atoms form polar covalent bonds the atom that ends up with the greater share of electrons is said to be oxidized while the.

. However additionally the two nitrogens each have one set of lone pairs and the oxygen molecule has two lone pairs open for hydrogen bonding. Theoretically there are a maximum of 5 water molecules that one urea molecule can hydrogen bond with but. Density functional theory DFT calculations are performed to study the conformations hydrogen-bonding network and the stabilities of all possible molecular associations ureaH 2 O n n 15 in aqueous solutions of urea.
This coordinate bond that nitrogen forms by donating its electron pair to the vacant orbital of other atom is how it can form 4 bonds. Popular Answers 1 Water molecule can haveform a maximum of four hydrogen bonds. Although urea has nearly three times the molecular volume of water the structure of liquid water is sufficiently open that a urea molecule appears to displace just two waters offering up to eight hydrogen bonds in place of the displaced pair.
Theoretically there are a maximum of 5 water molecules that one urea molecule can hydrogen bond with but there are 6 hydrogen. On average then each molecule can only form one hydrogen bond using its δ hydrogen and one involving one of its lone pairs. So for each nitrogen in urea there would be a hydrogen bond and for each hydrogen too.
Does urea have hydrogen bonds. One of the water hydrogen atom interacts with the urea oxygen atom through hydrogen bonding H 10 O 9 rOH 1876 Å. We review their content and use your feedback to keep the quality high.
Theoretically there are a maximum of 5 water molecules that one urea molecule can hydrogen bond with but there are 6 hydrogen bonds between one urea What is the maximum number of water molecules that in theory the. In this model the urea molecule is linked by one N HO and one O HO hydrogen bonds to water molecule Fig. Experts are tested by Chegg as specialists in their subject area.
I hope this helps. How many hydrogen bonds can urea form if dissolved in water. The vibrational spectra of urea also demonstrate such effects.
Two on the oxygen one on each Nitrogen and one on eachhydrogen. What unit of length would you generally use to measure a. The proposed molecular structure for the urea water mixture consists of a central urea molecules linked to five neighboring water molecules by six hydrogen bonds.
Clean-up recycling and disposal of macromolecules. Thus the maximum number of water molecules that can connect to urea is 8. Likewise the two nitrogen atoms can act as hydrogen bond acceptos because they each have one lone pair of electrons present.
Larger molecules have hydrogen bonding networks that contribute to specific high affinity bonding smaller molecules such as urea can also form these networks how many hydrogen bonds can urea form in water. See answer 1 Best Answer. What is the maximum theoretical number of water molecules with which in theory one urea molecule can hydrogen bond.
Playlist Organic Chemistry questions for practice. The other hydrogen bond O 9 H 4 N 2 is slightly longer rOH 2067 Å. Two given through the H atoms towards two other H2O molecules and two received on the O atom from H atoms of.
See the answer See the answer done loading. How many hydrogen bonds can urea form. In water there are exactly the right number of each.
In order to find the atoms that can form hydrogen bonds with the partial negative oxygen atom of a water molecule you must look for hydrogen atoms bonded to one of the three aforementioned electronegative elements. How many hydrogen bonds can urea Figure 2-26 form if dissolved in water. If you consider each molecule making 4 bonds then you are double counting each bond being made and accepted.
Smaller molecules such as. Finally the oxygen in the urea molecule may form hydrogen bonds with water as well but it has two lone pairs to donate so the oxygen atom may form hydrogen bond with 2 water molecules. But if you take 100 water molecules and count how many hydrogen bonds there are between them the answer will be about 200 because each molecules makes 2 bonds.
H N H OC I N H Figure 2-26 O 6 5 O 3 04. Which of the following choices best describes the role of the lysosome. The oxygen atoms of the CO groups each have two unshared pairs of electron and can form two hydrogen bonds with hydrogen atoms for a total of four hydrogen bonds.
N2 because of the oxygen atom of the urea4 because of the hydrogen. Question 33 1 pts Q026 Larger molecules have hydrogen-bonding networks that contribute to specific high-affinity binding. The hydrogen bond consisting of a hydrogen atom positioned asymmetrically between a nitrogen and an oxygen atom NHO plays a central role in the structure and.
Smaller molecules such as urea can also form these networks. Who are the experts. The B3LYP functional and the basis set 6-311Gd p are used through the calculations.
Follow answered Aug 12 2017 at 1638. This problem has been solved. You are right that the four hydrogens connected to the nitrogens could create a hydrogen bond with water.
In crystalline urea the oxygen atom participates in four hydrogen bonds 52. The other lone pairs are essentially wasted.
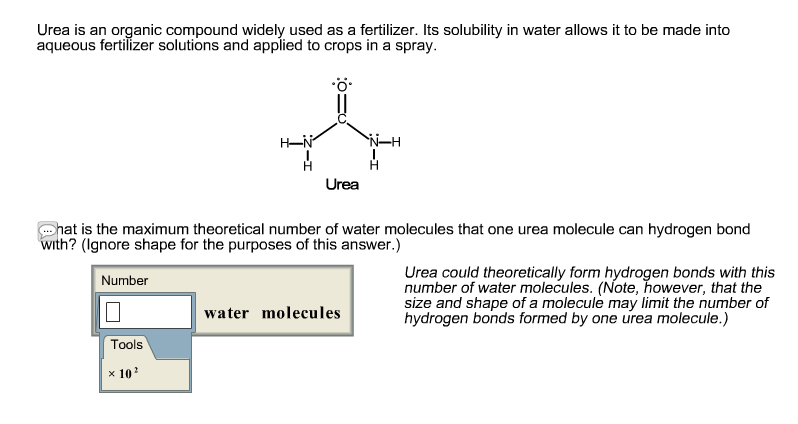
Solved Urea Is An Organic Compound Widely Used As A Chegg Com

Solvation Structure Of The Urea Molecule From Our Experiments One Of Download Scientific Diagram
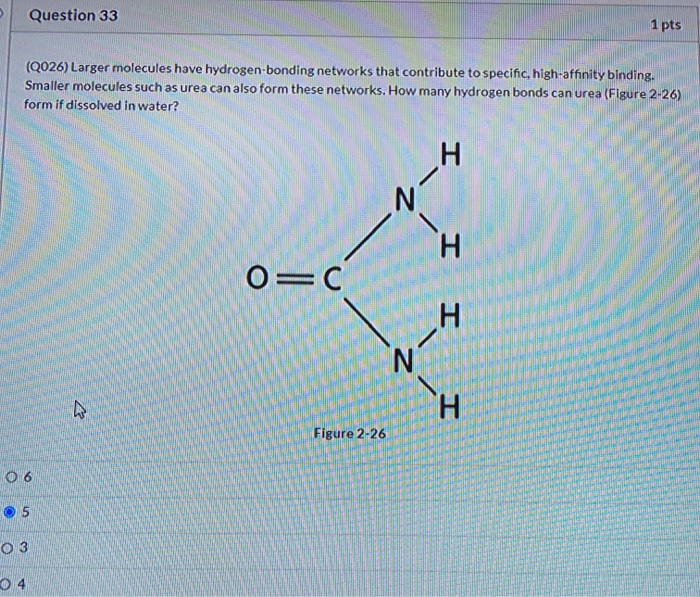
Solved Question 33 1 Pts Q026 Larger Molecules Have Chegg Com
0 Comments